Quality Assurance Consulting
Along with other services we also provide Quality Assurance Consulting to ensure your business operations operate smoothly and sustainably regardless of global conditions. This includes various assessments, implementations of strategies, and identification of quality matrix to ensure alignment with business needs, and goals, and continuous improvement.
RiverArk consultants with vast knowledge and experience assist clients by combining the experiences and abilities to find, develop, and implement the best solution for your company.
Our team of consultants delivers an ethical, unbiased, and compliant service with passion whether it is in the Pharmaceutical, Biotechnology, Medical Devices, or cosmetics fields.
Quality Assurance Consulting offering
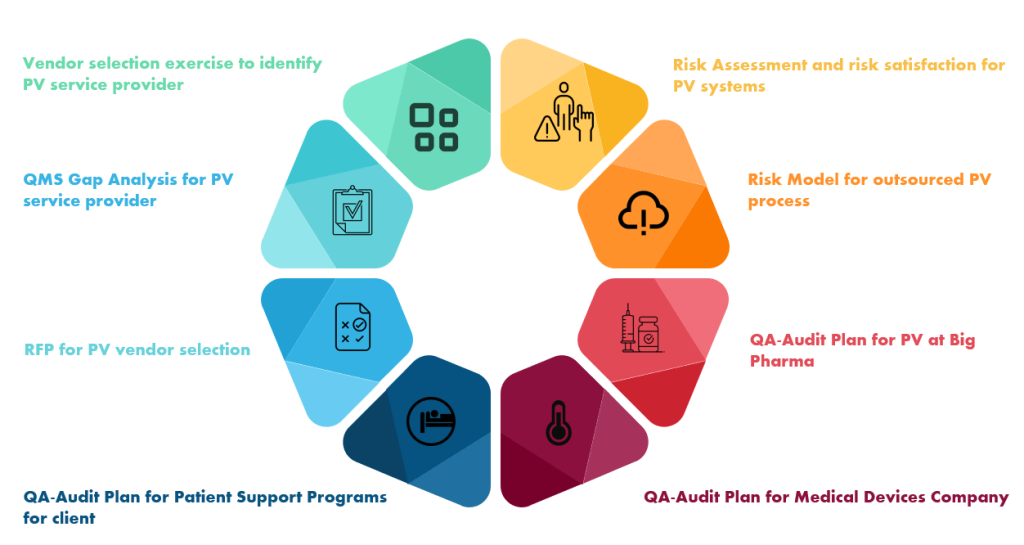
- Vendor selection exercises to identify PV service provider
- QMS Gap analysis for PV service provider
- RFP for PV vendor selection
- QA-Audit Plan for Patient Support Programs for client
- Risk assessment and risk satisfaction for PV systems
- Risk Model for outsourced PV process
- QA-Audit Plan for PV at Big Pharma
- QA-Audit Plan for Medical Devices Company
- Consultancy Services – QMS Evaluation, SOP Development